
When most people think of sea otters, they think about the cute and cuddly nature of the species. Whilst there is no denying their charming appearance, the species is so much more than just their looks. Believe it or not, sea otters are responsible for the balance and health of entire ecosystems. What many may not realise is the vital role sea otters play as a keystone species within kelp forest communities.
A keystone species is a species that plays a fundamental role within an ecosystem, influences the system, and thus, impact the ability of other species to survive within that habitat (Konar, 2000). Sea otters allow algal communities, such as kelp forests, to thrive by consuming, and therefore reducing the intensity of grazing pressures, caused by sea urchins (Kvitek et al, 1998). Sea otters are apex predator in kelp forests, meaning they sit at the top of the food chain in that community. Their predation upon sea urchins controls urchin abundance which in turns allows the kelp forests to thrive. Sea otters create kelp-dominant environments, which benefit other species that rely on lush kelp forests. For example, fish require kelp for feeding and as a nursery for their young, as well as larger marine species such as sea lions, which utilise kelp forests for shelter during harsh storms (Larson et al, 2012). An urchin-dominant state, on the other hand, entails the destruction of kelp forests by sea urchins (Larson et al, 2015). Such destruction results in ‘urchin barrens’.
Evolutionary road
Sea otters are part of the mustelidae family, which includes, but is not limited to, badgers, minks and ferrets. This family is considered one of the most species-rich, in terms of ecomorphological diversity and evolved adaptations, which makes sense, considering the sea otter is the only modern mustelidae to rely on marine life, whilst all others have no dependence on aquatic regions (Koepfli et al, 2008). Sea otters are currently found in two predominant populations; one of them being in North America, along the West coast, with their range extending from California to the Gulf of Alaska. The other primary population residing not far away, in the Sea of Okhotsk and Bering Sea, just off the coasts of Alaska and Russia (Doroff and Burdin, 2015).
Sea otters are equipped with adaptations to allow them to withstand cold climates and enable them to break hard prey exteriors such as those of clams, crabs and sea urchin. To feed, they pound their prey against rocks they prop on their stomachs and pry open the external layer with their claws and prominent teeth, seen in the Figure 1. They also consume up to 30% of their body weight in food every day, in order to maintain efficient thermoregulation in cold water (Pierotti, 2013). However, perhaps their most extraordinary evolutionary trait, is their fur. Sea otters do not have blubber like other marine mammals to keep them insulated. Instead, they have the densest fur of any animal in the world (Larson et al, 2015). on average, adults have up to 1 million individual hairs per square inch across a majority of their body, which traps air and repels cold water, thus providing insulation (Costa and Kooyman, 1982). With their flexible physique and precise grooming techniques, they ensure cold water never touches their skin. However, there is one threat that sea otters were not able to evolve traits to defend themselves against…humans. It was their luxurious pelt that almost drove them to extinction, being hunted for their pelts during the global fur trade.

Figure 1: A Sea otter using its unique feeding technique, which utilises its sharp teeth and claws, in order to obtain its latest prey, a sea urchin
The furocious hunt for sea otters
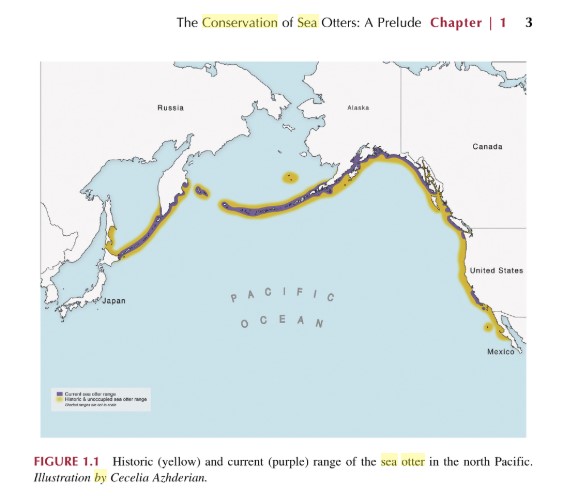
From as early as 1741 up until 1911, sea otters were hunted for their thick fur, during a period referred to as “the Great Hunt”, which entailed the killing, skinning and selling of sea otter fur, which was once considered an expensive commodity to own and wear (Ravalli, 2009). The mass demand for sea otter fur resulted in a 99% population loss during that 170-year period, with estimations that only a few hundred remained globally after hunting ceased (Larson et al, 2012). As previously mentioned, sea otter populations today are found around certain coastlines within the northern and eastern regions of the North Pacific Ocean. However, prior to hunting their range was much greater, stretching to coastlines around Mexico and Japan, as seen in Figure 2.
The Road to Recovery
As a result of conservation efforts, the International Fur Seal Treaty began protecting the surviving colonies as of 1911, and declared a cessation on “the Great Hunt” for all sea otters, just as they were on the edge of extinction (Doroff and Burdin, 2015). Following their protection, sea otter communities began to recover. Overall the population rose substantially within only the first few decades of the 20th century, from a mere few hundred to estimated tens of thousands of individuals (Davis et al, 2019).
Unfortunately, because the population was still so low and most communities were spatially segregated, this caused a population bottleneck and lack of genetic diversity, and along with it a plethora of health disadvantages (Ravalli and McGrann, 2019). Genetic diversity is generally high among species with prolonged lifespans and ensures the robustness to the next generation. By the end of the 20th century, when scientific research uncovered these genetic bottlenecks, translocation projects were implemented. These intervention measures not only re-located species to areas where they once resided before “the Great Hunt”, but also helped aid in the mixing of genetic diversity (Larson et al, 2002). If not for these conservation measures, many kelp ecosystems today would be negatively impacted due to the absence of sea otters, or cease to exist at all, as a result of their vital role as keystone species…perhaps that is why sea otters are referred to as ‘Keepers of the kelp’.
References
Costa, D. and Kooyman, G. (1982). Oxygen consumption, thermoregulation, and the effect of fur oiling and washing on the sea otter, Enhydra lutris. Canadian Journal of Zoology, 60(11), pp.2761-2767.
Davis, R., Bodkin, J., Coletti, H., Monson, D., Larson, S., Carswell, L. and Nichol, L. (2019). Future Directions in Sea Otter Research and Management. Frontiers in Marine Science, 5.
Doroff, A. & Burdin, A (2015). Enhydra lutris. The IUCN Red List of Threatened Species 2015: e.T7750A21939518. http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T7750A21939518.en.
Estes, J., Doak, D., Springer, A. and Williams, T. (2009). Causes and consequences of marine mammal population declines in southwest Alaska: a food-web perspective. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1524), pp.1647-1658.
ESTES, J., TINKER, M. and BODKIN, J. (2010). Using Ecological Function to Develop Recovery Criteria for Depleted Species: Sea Otters and Kelp Forests in the Aleutian Archipelago. Conservation Biology, 24(3), pp.852-860.
Hay, M., Parker, J., Burkepile, D., Caudill, C., Wilson, A., Hallinan, Z. and Chequer, A. (2004). Mutualisms and Aquatic Community Structure: The Enemy of My Enemy Is My Friend. Annual Review of Ecology, Evolution, and Systematics, 35(1), pp.175-197.
IUCN Red List of Threatened Species. (2019). The IUCN Red List of Threatened Species. [online] Available at: https://www.iucnredlist.org/species/7750/21939518 [Accessed 17 Sep. 2019].
Jessup, D., Miller, M., Ames, J., Harris, M., Kreuder, C., Conrad, P. and Mazet, J. (2004). Southern Sea Otter as a Sentinel of Marine Ecosystem Health. EcoHealth, 1(3).
Koepfli, K., Deere, K., Slater, G., Begg, C., Begg, K., Grassman, L., Lucherini, M., Veron, G. and Wayne, R. (2008). Multigene phylogeny of the Mustelidae: Resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biology, 6(1).
Konar, B. (2000). Limited effects of a keystone species: trends of sea otters and kelp forests at the Semichi Islands, Alaska. Marine Ecology Progress Series, 199, pp.271-280.
Kvitek, R., Iampietro, P. and Bowlby, C. (1998). SEA OTTERS AND BENTHIC PREY COMMUNITIES: A DIRECT TEST OF THE SEA OTTER AS KEYSTONE PREDATOR IN WASHINGTON STATE. Marine Mammal Science, 14(4), pp.895-902.
Larson, S., Jameson, R., Etnier, M., Fleming, M. and Bentzen, P. (2002). Loss of genetic diversity in sea otters (Enhydra lutris) associated with the fur trade of the 18th and 19th centuries. Molecular Ecology, 11(10), pp.1899-1903.
Larson, S., Jameson, R., Etnier, M., Jones, T. and Hall, R. (2012). Genetic Diversity and Population Parameters of Sea Otters, Enhydra lutris, before Fur Trade Extirpation from 1741–1911.
Larson, S., Bodkin, J. and VanBlaricom, G. (2015). Sea otter conservation. 1st ed. p.2-43.
Ling, S., Scheibling, R., Rassweiler, A., Johnson, C., Shears, N., Connell, S., Salomon, A., Norderhaug, K., Pérez-Matus, A., Hernández, J., Clemente, S., Blamey, L., Hereu, B., Ballesteros, E., Sala, E., Garrabou, J., Cebrian, E., Zabala, M., Fujita, D. and Johnson, L. (2015). Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1659), p.20130269.
Nichol, L. (2015). Conservation in Practice. Sea Otter Conservation, chapter 13 pp.369-393.
Paine, R. (1980). Food Webs: Linkage, Interaction Strength and Community Infrastructure. The Journal of Animal Ecology, 49(3), p.666.
Ravalli, R. (2009). The Near Extinction and Reemergence of the Pacific Sea Otter, 1850-1938. The Pacific Northwest Quarterly,100(4), 181-191.
Ravalli, R. and McGrann, M. (2019). Sea Otter Hunting and Conservation in Southern California since the Gold Rush. Southern California Quarterly, 101(3), pp.265-284.
Thometz, N., Kendall, T., Richter, B. and Williams, T. (2016). The high cost of reproduction in sea otters necessitates unique physiological adaptations. The Journal of Experimental Biology, 219(15), pp.2260-2264.



Recent Comments