This post has been contributed by Dr Michelle Rourke, CSIRO Fellow and a member of the Law Futures Centre and Dr Mark Eccleston-Turner, Keele University
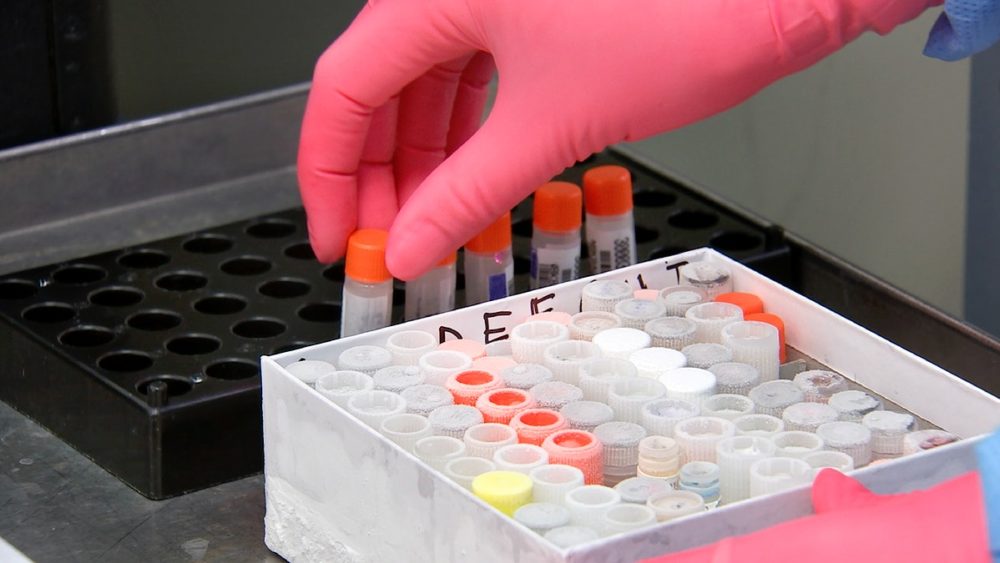
On the 24 May 2021, the World Health Organisation (WHO) announced that arrangements had been made “to launch the first WHO BioHub Facility” in Spiez, Switzerland. The BioHub would be the first of what is projected to be a global network of laboratories that will exchange SARS-CoV-2 virus samples, the causative agent of COVID-19, and samples of “other emerging pathogens”.
Throughout the COVID-19 pandemic, scientists have monitored the spread of SARS-CoV-2 and witnessed the emergence of SARS-CoV-2 variants of concern. Scientists require access to up-to-date SARS-CoV-2 virus samples, isolated from patient specimens, to ensure the available COVID-19 vaccines will effectively protect populations from novel variants of concern and to conduct further research and development.
When a virus is isolated from a patient specimen, scientists can determine the genetic sequence of the virus sample and see how it is related to the other viruses that came before it. Scientists then upload the genetic sequence data of the virus to a sequence databank so that other scientists have easy access to the data and can use it to conduct their own research. Exchanging virus samples, genetic sequence data and epidemiological information has been vital to the pandemic response, including making diagnostics and vaccines.
The objective of the WHO’s new BioHub in Switzerland is to “enhance the rapid sharing of viruses and other pathogens between laboratories and partners globally”. According to the WHO’s announcement, made during the recent World Health Assembly in May this year, “the [BioHub] facility will serve as a centre for the safe receipt, sequencing, storage and preparation of biological materials for distribution to other laboratories, in order to inform risk assessments, and sustain global preparedness against these pathogens.”
The problem the BioHub is seeking to solve is that the bilateral sharing of pathogen samples between laboratories in different countries can be slow, difficult to coordinate and expensive. So, the BioHub would conduct these activities in a multilateral manner, with many countries providing their SARS-CoV-2 samples to the WHO’s BioHub, and the WHO distributing those samples of interest to scientists in other countries upon request.
Coordinating the multilateral exchange of virus samples is not a new activity for the WHO. In 2011, WHO member states adopted the Pandemic Influenza Preparedness (PIP) Framework for the sharing of influenza viruses and access to vaccines and other benefits. This non-binding international instrument was retrofitted to an existing international network of influenza laboratories that had been exchanging influenza viruses with each other since the 1950s to monitor ever-changing strains of influenza virus as they infect human and animal hosts across the globe.
The PIP Framework asks that the recipients of samples of influenza viruses with human pandemic potential contribute some of the benefits of research and development to the WHO. In the event of an influenza pandemic, the WHO will distribute these benefits to countries in need. For example, vaccine manufacturers that use influenza virus samples obtained from the WHO’s laboratory network can elect to donate 10% of real time pandemic vaccine production to the WHO, or reserve those doses for the WHO to purchase at a reasonable price. This is a transaction known as “access and benefit-sharing”, which is short for access to genetic resources (virus samples, in this case) and the sharing of benefits associated with their use.
The PIP Framework only applies to influenza viruses with human pandemic potential. It doesn’t even apply to seasonal influenza viruses, where there is no WHO-coordinated obligation to share any of the benefits of research and development. The same goes for the genetic sequence data from influenza viruses with human pandemic potential. If a pharmaceutical company can make a product using genetic sequence data instead of the physical sample, they have effectively avoided the benefit-sharing obligations that would have existed under the PIP Framework.
Pathogen access and benefit-sharing is an active topic of research and debate for the WHO and its member states. The tension essentially comes down to the fact that lower- and middle-income countries (LMICs) are often expected to share their pathogen samples and associated genetic sequence data without restrictions so that companies can make vaccines and other medicines. Meanwhile, it is the high-income countries (HICs) that benefit most from this arrangement. They often get the raw materials to make the vaccine for free and they get first dibs on all the vaccine doses. The COVID-19 pandemic has highlighted this starkly.
What made the announcement of the BioHub so surprising was that it was devoid of information about access and benefit-sharing arrangements. It did, however, explicitly state that the “WHO will broaden its BioHub System for the use of biological materials by qualified entities – such as manufacturers – for the development of medical by-products for fair allocation to countries”. There are legitimate concerns that the WHO BioHub is therefore prioritising the access side of the access and benefit-sharing equation. Securing access to pathogen samples for researchers but remaining silent on whether or not the BioHub will secure any associated benefits for the countries contributing those samples.
The inequitable distribution of COVID-19 vaccines and the fact that the pandemic has exacerbated pre-pandemic healthcare disparities clearly “forecast potential ethical issues for COVID-19 biobanking”. Concerns about access and benefit-sharing were entirely foreseeable and yet the BioHub was announced without any attempt to address these concerns. Furthermore, this arrangement was made by the WHO without broad consultation with its member states, and this top-down approach to the BioHub’s implementation further undermines the trust of LMICs, whose participation would be critical to the proper functioning of the BioHub. The health disparities that have left LMICs dangerously unprotected from the COVID-19 pandemic also contribute to the fact that LMICs will likely be the most important source of SARS-CoV-2 variants of concern in the future.
Countries have sovereignty over pathogen samples that originate in their territories under the United Nations’ Convention on Biological Diversity and its Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (Nagoya Protocol). This means it is entirely up to them whether they choose to provide samples to the international scientific community, and on what terms, whether that be through bilateral arrangements with interested parties (such as research institutions or pharmaceutical companies), or through multilateral initiatives like the BioHub or the PIP Framework.
For the BioHub to succeed, the WHO needs to seriously engage with LMICs to ensure that their concerns about the fair and equitable sharing of the benefits arising from research and development are addressed. We can no longer expect LMICs to continue to provide, free of charge, one of the few bargaining chips they have in a deeply unfair global system that continues to prioritise the health of people in HICs over the lives of people in LMICs.



Recent Comments